What About the Vaccine's Ability to Prevent plain COVID-19?
Short Term Side Effects Versus Catching Non-Severe COVID-19 Still Not Favorable Risk Calculus For Taking the Vaccine
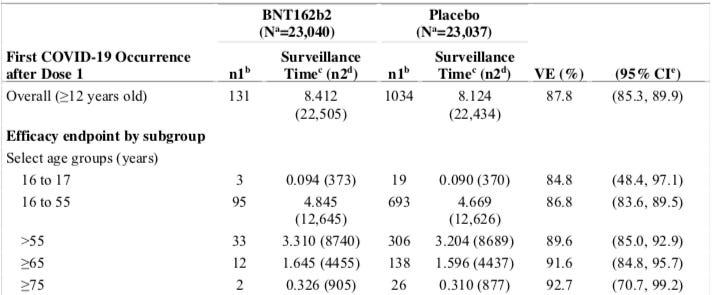
The real world efficacy from the initiation of dose 1 in preventing RT-PCR confirmed COVID-19 is given in the table below from Pfizer’s preprint reporting the 6 month followup. Updated data does not appear on the label for this measure:
Source: SUPPLEMENTARY APPENDIX to Six Month Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine, Page 13, Table S5
This represents a 1,034/22,434 = 4.6% rate of disease in the placebo arm versus a 131/22,505 = 0.6% in the control arm. If you subtract the true numbers, that implies a treatment benefit in 4.0% of patients. In terms of annual average risk that would be 131 cases / 8,412 patient years = 1.6% in the vaccine arm versus 1,034 / 8,124 = 12.7% in the placebo arm, for an annual difference of 10.1%. Note, whilehelpful in normalizing for slight differences in follow up times, I do not really like annualizing the numbers like this because we have no proof the treatment effect is durable up to 12 months. Indeed there are some very strong reasons to believe it would not based on the rate of viral mutations in coronaviruses. We really only have an average of 12*8,412/22,505 = 4.49 months in the vaccine arm versus 12*8,124 / 22,434 = 4.35 months in the control group. And since the trial is now unblinded and crossed over, that is all we will ever have! Most of the people in the trial have probably been vaccinated by now.
But the treatment benefit is real and very highly statistically significant with a risk reduction of 87.8% with a confidence interval of 85.3% to 89.9%.
Some people might point to the 4.0% absolute differential benefit in the vaccine arm in terms of preventing COVID-19 as a reason to get the vaccine, despite the 41.6% increased risk in severe outcomes shown in the trial. In fact, there are many who say that as the 0.4% absolute risk over the course of the study (0.9% annualized risk adjusted for different follow up times) is just too small to worry about. And some people are naturally predisposed to discount low probability events.
First of all, most people are afraid of COVID-19 because they are afraid of getting severe COVID-19 or becoming a long hauler. It would have been nice for Pfizer to have collected some information on duration of disease in both arms to understand how frequent this long hauler phenomenon is and what extent we could prevent that kind of disease. We have no such data. But if you are looking to avoid death from COVID-19 or severe COVID-19, the only data we have, we know the decision calculus makes no sense because the difference in severe adverse events in the vaccine arm is much higher. So if you do not want to get COVID-19 because you are worried about it becoming severe or dying, the numbers just do not support that risk-reward calculus, quite the opposite.
Now if I just focus on the short term benefit of 4% in terms of non-severe COVID-19, everyone looks at things differently. To me that 0.4% absolute risk of a severe outcome, roughly 1 in 250 is not exactly small. In fact, it is over three times higher than the absolute risk of getting severe COVID-19 by itself in the placebo group, a factor that was already factored into this 0.4% absolute rate. Let’s remember these are severe adverse reactions, which could easily include events like the Sally Kirkland case that are not life-threatening but very painful. I will suffer through COVID-19 and even severe COVID-19 to avoid that. That 1 in 250 sounds like low odds until you are the person that loses that lottery, when you are an otherwise healthy person.
But lets say small probabilities do not bother you, as I have heard from many people. How about large probabilities? Let’s compare the non-severe adverse events to the non-severe COVID-19 cases so again we have an apples to apples comparison.
As you can see in the Tables below, there are tons of side effects from the vaccine just from the 7 day windows after taking the vaccine. This table in particular has smaller patient numbers because it is a phase 2/phase 3 substudy. This data was not collected on every patient; it was “solicited” from a prospective survey to patients to analyze their short term reaction to the vaccine since many of these events are likely to go unreported. We have to use the percentages to make things meaningful across the whole population of the trial. Also note, the majority of these events will not appear in “unsolicited adverse events” numbers we will discuss later.
Source: COMIRNATY Package Insert, Pages 8-9, Table 2: Study 2 —Frequency and Percentages of Participants with Solicited Systemic Reactions, by Maximum Severity, Within 7 Days After Each Dose - Participants 16 Through 55 Years of Age - Reactogenicity Subset of the Safety Populaton
Source: COMIRNATY Package Insert, Pages 11, Table 4: Study 2 — Frequency and Percentages of Participants with Solicited Systemic Reactions, by Maximum Severity, Within 7 Days After Each Dose - Participants 56 Years of Age and Older - Reactogenicity Subset of the Safety Populaton
I want to focus on one line item, use of pain medications and anti-pyretics. To me that is a very good statistic to encompass how bad the reaction actually was. If I get a jab and get a little pain from the injection site short term, that is not a big deal. I am not going to take a pain killer for that.
However, whatever reaction I have, and you can see there are tons to chose from – muscle pain, joint pain, headache, etc. – I am not going to go out and take a pain medicine or an antipyretic unless is it really uncomfortable, some would say to the same extent as if you just got sick. I thus consider this a proxy measure for true intensity of the reaction to the vaccine.
As you can see if we add together the two age groups, 16 to 55, and 56 and up there are 1213 + 688 = 1,901 patients or 41.9% (1,901 / (2682+1860)) of the sample in the vaccine arm needing to use these treatments 7 days after being jabbed the second time. Nearly half… This compares to a background reaction of 320+170 = 490 in the placebo arm or 10.8% (490/(2684+1833)). So if you take the difference that is a 31.0% absolute risk over baseline of needing to take pain meds or antipyretics with 7 days after a vaccine. By taking this vaccine are giving yourself a 1 in 3 chance of feeling sick right after taking the jab in addition to that 1 in 250 chance of a severe outcome!
By the way, this is just for the second jab. There is also a risk on the first jab that is not insignificant either. For the first jab that is 382 + 805 = 1,187 patients for a rate of 24.2% (1,187 / (2899+2008)) in the vaccine arm. For the control you have 398+224 = 622 patients for a rate of 12.7% (622/(2,908+1,989). This is a 11.5% differential risk, roughly a third of the second dose. Please consider the fact the ratio is getting worse for each jab when you go for that third jab, when we have no controlled data at all on how much worse that reaction might be.
If we consider the probabilities to be independent for each jab, we can calculate the odds of having to use pain medication in either of the jabs over baseline. There probably is some correlation between jabs, but we do not have the data to say so one way or the other. If there is a correlation then probability goes down but the expected number of events per person goes up. But using the aforementioned assumption, the probability of needing to use pain medicine or antipyretics within 7 days of the jab is one minus the probability that you do not get a reaction in either jab = 1-(1-0.419)*(1-.242) = 55.9%. This compares to 1 - (1 - .108)*(1-.127) = 22.2%. So the relative risk is 2.5 and the absolute risk is just a little higher than dose 2 alone at 33.7%.
That is just the short term reaction. We already covered the severe adverse events, but there were also non-severe side effects that were probably more comparable to a mild case of COVID-19.
Source: SUPPLEMENTARY APPENDIX to Six Month Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine, Page 11, Table S3
This was 6,617 in the vaccine group (30.2%) versus 3,048 in the control group (13.9%). That is roughly a 16.3% absolute risk of getting any adverse event when you take the vaccine versus control. While not directly comparable to all the other numbers we are using as the label data was much more incomplete, these adverse event numbers were updated on the label:
Source: COMIRNATY Package Insert, Pages 12, Unsolicited Adverse Events
The updated numbers are 6,947 unsolicited adverse events (4,396 + 2,551) in the vaccine arm for a 31.7% adverse event rate (6,947/*(12,995+8931)). In the control you have 3,568 unsolicited adverse events for a 16.3% event rate (3,568/(13026+8895)). This represents a 1.9 relative risk and 15.4% absolute risk, which is slightly less but in the same ballpark. And remember, this does not include the “solicited adverse events” such as the 33.7% risk of needing to take a pain medication identified in the substudy. The other patients were not asked about it, and there would only be overlap when they happened to report the underlying symptom causing their pain to investigators.
Here is a table showing the event rates and absolute negative risks represented by the differences in rates between arms for the vaccine and control groups below, categorized by severity:
How does that 4% or 1 in 25 feel now? Still thinking that getting the jab is a good idea?
And we have all heard stories about people being laid up for one or two days from taking the jab. I have been on several live streams where one person was conspicuously absent because he is feeling sick from the jab. The anecdotal evidence matches the clinical data if we use the pain/antipyretic medication as a proxy for practical illness after taking the jab.
Now certainly you can argue that a COVID-19 case can be more prolonged, and I completely agree with that. If the rates were equal or just close and there was no issue with severe outcomes, I would say that COVID-19 is more severe and needs to be weighted more heavily and the shot could be justified.
The problem is we are looking at an 8-fold increase in probability of negative effect just looking at the pain medicine input, before we talk about the 15%-16% general adverse event rate. You are comparing much more certainty with potentially more severity. But you are also doing that on severe events! So if you value severity over certainty, it makes sense to be worried about the severe events, and if you value certainty over severity, you would be more worried about the near term after effects of the jab in addition to the overall adverse event rate.
But this is an individual call that everyone has to make. I personally want to avoid the 0.4% absolute risk of increased severe events and do not like the fact that on top of that I am giving myself a 1 in 3 chance of feeling like crap right after the jab when I am virtually never sick because I regularly take active measures to stay healthy. The way I look at it, either at moderate events, severe events, or mortality, the vaccine data is worse on every level (even when we talk about deaths, while not significant, there are still 4 more deaths in the vaccinated arm versus control according to updated data buried off the label at the FDA website).
The only real negative I see to not taking the vaccine, is wannabe Nazi governments trying to restrict your access to society and commerce unless you submit to the experimental and potentially dangerous jab.
That is something people must resist to the death though, because regardless of the outcomes on the trial, it is better to die a free man than to live as a slave.