41.6% Increased in Risk of Adverse Severe Outcome Over Control When You Take the Vaccine Including All Severe COVID-19 Cases
Pfizer's own trial data show a bleak risk reward picture for COMIRNATY
You do not need to advocate for some crazy conspiracy theories on what may or may not be in the vaccine to know that taking it is, scientifically speaking, a bad idea. You only have to look at Pfizer’s own data. No one is more pro COVID-19 vaccine than Pfizer investigators. While they might have an incentive to make their data look better than it really is (not properly counting severe COVID-19 cases), they have no incentive to lie in a manner than makes their vaccine look worse than placebo. We should thus be able to use it without the establishment saying their data is “fake news.”
22.084 cases per 1,000 patient years. 31.263/22.084 = a 1.416 relative risk or 41.6% increased risk of adverse event relative to placebo. The absolute risk is 31.263 - 22.084 = 9.179 cases per 1,000 patient years or 0.92% per year.
Pfizer’s own data shows that you have a 41.6% increased risk of an adverse severe outcome including severe COVID-19 cases versus placebo. This is 31.3 events per 1,000 patient years versus 22.1 events per 1,000 patient years, for an overall negative risk of a severe outcome from taking the vaccine at 0.92% per year. This is actually the most accurate way of viewing the risk because it normalizes for different follow up times in each arm.
In terms of the percentages of each arm in the trial it was 1.2% in the vaccine arm versus 0.8% in the control arm. This is highly statistically significant based on a Clopper-Pearson analysis I did in Excel (p-value of roughly 0.006). This is the same methodology specified by Pfizer in their preprint of the six month results, and makes sense given you expect low probabilities for individual events. I can also why see why they did not provide p-values on the comparisons in their tables. 1.2% risk (a 1.4% to 1.1% 95% confidence interval) in the vaccine arm versus 0.8% (a 0.9% to 0.7% confidence interval) in control, for a 0.4% absolute excess risk of taking the vaccine when just comparing the two arms over the course of the trial.
The Details
Let me take you through the exact numbers and risk calculus, since I do not expect people to take my numbers without providing proof as to how I got them.
Deaths - No Meaningful Difference with Deaths Barely Higher in the Vaccine Arm
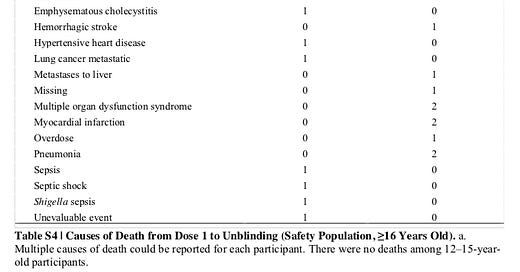
Let’s start with deaths to get that out of the way. Everyone is saying we have to take the jab to “save lives.” Well based on data released from the pre-print of the COMIRNATY 6 month results, there were more deaths in the vaccine arm compared to the placebo arm: 15 versus 14.
Source: SUPPLEMENTARY APPENDIX to Six Month Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine, Page 12, Table S4
This is statistically meaningless. COVID-19 deaths were also statistically meaningless: 1 in the vaccine arm versus 2 in the control. This is data from the preprint so there might be more up to date data now. But I did not see the deaths listed in the label. I did a search for the word “death” just to make sure, and it only appears twice on page 18 when discussing the definition of severe COVID-19 discussed in my prior article on the under reported severe COVID-19 case incidence on the BLA label. That is probably not a coincidence given it contradicts the whole “we need the vaccine to save lives” angle everyone is pushing.
Those two deaths in the placebo arm represents a 0.009% mortality rate (14/21,921) from COVID-19 over an average 4.3 months of followup. To put that in perspective, your lifetime risk of being hit by lightening is listed as 0.007%. I calculate the 95% confidence interval on that as between 0.0179% and 0.004%. Not exactly scary. Certainly not worth shutting down the whole world and implementing some Nazi/USSR/666 style vaccine passport system.
At a presentation at the FDA panel meeting in December, the CDC estimates (or it might have been FDA, I don’t remember) for death risk every 6 months was 1/1000, which implies an annual risk of 0.2% and an expected number of deaths in the placebo arm of 16 based on the 8,124 patient years of follow up (see Table S5, page 13 of supplementary data below; 8,124 /500 = 16.25). The annualized risk as demonstrated from this trial was nearly a full order of magnitude lower than the 0.2% agency estimate at what I calculate to be 0.025% (2 events / 8,124 patient years).
Source: SUPPLEMENTARY APPENDIX to Six Month Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine, Page 13, Table S5: Vaccine Efficacy Overall and by Subgroup after Dose 1 During the Blinded Placebo Controlled Follow-up Period (All-Available Population)
Again, this is Pfizer’s randomized controlled study, not numbers from some shady anonymous source. This is probably the best mortality rate we have for the general population because Pfizer presumably has the most data being the first to receive a BLA. Also, remember that those least likely to die from COVID-19, those below 16 years of age, were excluded from the trial. So, if you believe the enrolled patients are truly representative of the general population, that 0.025% annual mortality risk is likely too high for people of all ages.
This is what we shut the world down for. The Chinese Communist Party has to be laughing their asses off right now. This will probably go down as the greatest psychological operation in human history. And if you still think that release was still an accident, and all those mass fatalities from Wuhan the CCP streamed into your living room were both real and from this coronavirus, and not something else, I have some beautiful swamp land in Florida to sell you. It is conceivably possible that the virus just weakened a lot, as viruses tend to do since killing your host quickly is not a good survival strategy; but, given everything else we know about this outbreak, that is not the most likely story at all. China benefited by far more than anyone else. It is a Chinese New Year’s gift the CCP probably continues to celebrate given it solved their trade war problem.
But, back to the data. Even though there is a very meaningless difference in COVID-19 deaths, I concede that difference could become meaningful, statistically speaking, over time if you believe the vaccine works. And the efficacy in preventing all RT-PCR confirmed COVID-19 is about as certain as you can get. But in absolute risk differential, it not likely when you are starting with a 0.009% sample for mortality in the placebo arm.
Indeed people who are most likely to die, generally are people who are least likely to have a well functioning immune system to begin with. This could lead to lower than expected decreases in mortality than the 91% reduction in common disease.
And even if you saw a difference over time in COVID-19 deaths with larger patients, you have no way of knowing if the side effects themselves would offset this with small numbers of excess deaths from treatment complications. Indeed in this sample, you had three deaths in the vaccine group from adverse events versus five in placebo (see Table S3 later in article), in addition to the one COVID-19 death in the vaccine arm versus two in placebo. But this difference was made up largely by cardiovascular deaths of various types. There were 9 in the COVID-19 group versus 6 in the placebo arm (which I count as aortic rupture, arteriosclerosis, cardiac arrest, cardiac failure congestive, cardio-respiratory arrest, hemorrhagic stroke, hypertensive heart disease, myocardial infarction), a 50% increase. This is not statistically meaningful, like the other death data. Even the 4 to 1 cardiac arrest comparison by itself that is the big driver of this difference, by my math, is not statistically significant. However like with COVID-19 deaths, that does not mean it might not become statistically significant if many more patients were enrolled. And it is certainly conceivable this could be a result of the demonstrated low level cardiotoxicity of this vaccine.
The reality is, before most people ever heard of myocarditis being an issue seen with the vaccine, risk of myocarditis actually appeared in the original label published by the UK government (see my post “
“). So they noticed this effect early on and found it important enough to put it on the label in the UK. But it was not even in the FDA briefing document in December 2020 about the same time! There have also been reports of vaccine induced thrombocytopenia. For all we know, subclinical cardiac toxities (i.e. those that are not apparent immediately; like the original vessel wall injury that starts a process that causes an artery to clog over time) could eventually lead to modest elevation of the mortality rate over time, enough to potentially offset any speculative decrease in COVID-19 mortality over time. All of this is just speculation though in terms of reasons to believe there will be a mortality benefit or disadvantage long term.
But the bottom line is, there is no clinical proof that the vaccine prevents deaths at all. If a drug company tried to say that in their advertising, a competent FDA would slap them down hard for marketing a claim when there is no proof of that claim. Indeed, as of now, numerically there is 1 more death in the vaccine arm.
So mortality is not an argument for the vaccine at this moment, and potentially never based on a very low risk. We do have enough severe events to make a definitive statistical conclusion though.
Update (8/28/2021): Well actually the death comparison looks much worse now. We now know why the FDA did not put the deaths on the label. In an obscure document on the FDA website I found yesterday it was revealed the numbers of death in the vaccine arm were 21 versus 17 in control. These occurred all before April, so I have no clue how Pfizer presented the 15 versus 14 a few weeks ago in the data you see above.
This is still not statistically significant, but the idea this is a life saving vaccine is getting more absurd by the day.
Severe Adverse Outcomes - The Only Data You Need to Justify Not Taking this Vaccine
In the recent preprint of the 6-month data, Pfizer revealed the severe adverse event rate. Interestingly enough, this data was nowhere in the BLA label. As a result, I have a little less data than actually exists as, the number of non-severe adverse events reported in the label 6,947 (4,396 ages 16-55 +2,551 ages over 55; page 12 of the label under Unsolicited Adverse Events) was higher than in the preprint (6,617) despite representing a slightly smaller age group. That difference could theoretically simply reflect some additional solicited adverse events (i.e. surveys) that were not included in the label but appear in Table S3 which does not qualify the AE as “unsolicited,” but I assume that is an oversight because the reactogenecity study they conducted should have raised those numbers quite a bit if solicited events were included. I assume the severe adverse events in both arms are higher as well from the inclusion of more patient years of data, but have no way at the moment of knowing.
Source: SUPPLEMENTARY APPENDIX to Six Month Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine, Page 11, Table S3
Based on what we have the number of severe adverse events was 262 (1.2%) in the vaccine arm versus 150 (0.7%) in the placebo arm (page 11, Table S3). There are some more adjustments we will need to make later in this article to account for some differences in total patient years to be totally rigorous and generate incidence rates, but this is the easiest way to interpret the data. I calculated the confidence interval as 1.3% to 1.1% for the vaccine arm and 0.8% to 0.6% for the placebo arm with a p-value of roughly 0.0001. That means there is a 99.9999% chance the vaccine arm has more severe adverse events than control. In other words, this does not appear to be a fluke of chance.
Table: Calculation of Clopper Pearson Confidence Intervals On Excel Spreadsheet For Severe Adverse Event Rate
If we look at severe COVID-19 cases, we have one in the vaccine arm versus 30 in the placebo arm.
Source: SUPPLEMENTARY APPENDIX to Six Month Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine, Page 15, Table S6
Please note that this is somewhat different than the data on the label. There are several reasons why. First I want to match in time all the data on the severe adverse events in Table S3 since we do not even have severe adverse event data on the label to compare. The one problem I could not get around is the efficacy data here also includes 12 to 15 year olds which are not addressed on the table of adverse events. Since the severe adverse event table is heavily against the vaccinated arm. From an absolute numbers perspective, since the adverse events heavily are against the vaccine arm, discrepancy should theoretically benefit the vaccine versus placebo in the comparison. Finally, since side effects are evaluated from dose 1, so should efficacy for an accurate portrayal of real world efficacy, not the endpoint Pfizer chose because it thought it would make the product look better. So actually I am again giving them a better differential result than they show on the label for the predefined timeframe for efficacy (7 days after the second dose) as per FDA protocol (CDC criteria data is not shown in this report). As you can see in Table S6, in this matched patient group that would look slightly worse for Pfizer at 1 versus 23 if I used the timeframe they originally chose.
In reality that should be at least 2 in the vaccine arm, since Pfizer does not appear to have counted that COVID-19 death as “severe” like it was required to under protocol. However, I am not adding that back because I assume that those are presumably already counted in the severe adverse event group. Also there should be the two “suspected COVID-19” cases in the vaccine group we know about who were hospitalized but did not count as “COVID-19” because there was no positive PCR test (see VRBPAC 12/10/2020 Briefing Document, page 42, Suspected COVID-19 Cases). But this is Pfizer the same group that did not seem to think a COVID-19 pneumonia death was severe COVID-19, so you never know…
Also note that it is possible that some severe COVID-19 cases could be counted under severe adverse events as well. I cannot know for sure as I have not seen the exact criteria for classification as “severe adverse event.” But if there were overlap in the numbers we are combining, that would only raise the risk for the vaccine since most of the severe COVID-19 cases are in the placebo group.
So if you add those cases together for total risk of severe event you get 262+1 = 263 out of 21,926 (1.2% approximately) in the vaccine arm for and 30 + 150 = 180 out of 21,921 (0.8% approximately) in the placebo arm. That is a 50% increase in relative risk and a 0.4% increase in absolute risk over a 4.4 month period when comparing the raw event rates in both arms over the study. Here is the data on confidence intervals:
Table: Calculation of Clopper Pearson Confidence Intervals On Excel Spreadsheet For Severe Adverse Outcome Including Severe COVID-19
This is just a rough back of the envelope calculation that is easier for most people to follow and gets you pretty close to the more scientifically valid numbers in terms of relative risk (which works out to 41.6%, not 50%).
Refining the Calculations
We still have to make some minor adjustments to these numbers because there is a mismatch in patient numbers and follow up in both the adverse events and efficacy tables as well as between the placebo and vaccinated arm. To get apples to apples numbers we have to look at the incidence per 1,000 patient years in both groups instead of using just the raw numbers.
For the severe adverse events (SAEs) you have 262 SAEs in the vaccinated arm in 8,412 patient years (see table S5 above) which represents 262*1,000/8,412 = 31.145 cases per 1,000 patient years. In the placebo arm you have 150 SAEs in 8,124 patient years for 150*1,000/8,124 = 18.464 cases per 1,000 patient years. This represents a 68.7% increased risk of a severe adverse event by getting the vaccine.
For the severe COVID-19 numbers, there was 1 case in the vaccinated arm through 8,439 patient years for 1*1,000/8,439 = 0.118 per 1,000 patient years incidence. For the placebo arm you have 30 cases in 8,288 patient years for a 30*1000/8,288 = 3.620 cases per 1,000 patient years.
So if we add that up in the vaccine arm: 31.145+0.118 = 31.263 total incidences per 1,000 patient years and in the control arm: 18.464 + 3.620 = 22.084 cases per 1,000 patient years. 31.263/22.084 = a 1.416 relative risk or 41.6% increased risk of adverse event relative to placebo. The absolute risk is 31.263 - 22.084 = 9.179 cases per 1,000 patient years or 0.92% per year.
In reality, things are somewhat more complicated than this. You generally annualize things out to normalize for the differences I highlighted above. In reality, the adverse events will tend to be more front end loaded with a vaccine so annualizing can overstate the harms. The vaccine efficacy cannot really be annualized either because there is no evidence yet that the response is durable beyond the 4.5 months presented because this is the data we have. And we will not have good data on that going forward because Pfizer encouraged people to cross over and the study has been unblinded.
Generally speaking when looking at a rigorous comparison between arms like this, you would also want to account for when something happens, weighting events closer to the treatment more than events further away. This is the way most survival and time to some sort of morbidity event trials are done. If you think about it, it makes sense. You prefer adverse events, particularly death, to be as far away as possible and are not necessarily just interested in the raw rate at a given timeframe in the future. We do not even have access to the data to do that. But that sort of analysis, as you might suspect, would be worse for a vaccine where events tend to happen happen early.
The fact the establishment is now telling people they need a “third jab” relatively close to the first two after a bunch of highly vaccinated countries are getting crushed by COVID-19 does make annualizing data a little more applicable. If you have to keep jabbing people over a course of treatment then the side effects are not entirely front end loaded at all and will become periodic, with perhaps efficacy that is maintained. If you assume that is correct, then the incidence model presented by Pfizer in their primary endpoint and in our incidence analysis here is a reasonable approximation of the relative risk going forward.
However, I doubt third jabs of the same vaccine not modified to account for mutant variants are really that useful for anything except delaying potential antibody dependent enhancement (a subject for another article). You also have to remember that adverse reactions went up after the second dose in the trials. We have no idea what will happen with the third dose. If it continues the same trend, you would expect the side effects to get worse over time with each subsequent injection.
We are in completely unchartered territory here with the entire population duped into being guinea pigs probably have no idea what to expect going forward. This is why you do long term studies before you widely distribute a vaccine to the general population.
Comparing Event Severity
Now you might be saying “but a severe COVID-19 case is not the same as a severe adverse event.” And that is undeniably true. We really need details on every single severe COVID-19 case and every single severe adverse event to be able to even attempt to compare them. We simply do not have that data, only the category “severe” which is not even well defined for adverse events like it is for severe COVID-19 since that was a pre-specified secondary endpoint for the trial. And just to be clear, I am not saying Pfizer did not define those well, just that we are not presented with that exact definition in the clinical data reports I have referenced or the BLA label.
“Severe” is generally a measure of intensity of a symptom, and “serious” or “critical” generally involves a life threatening situation. It is a little more complicated than that and the FDA has general guidance on what is “serious,” but I have no idea how Pfizer actually defined “severe” versus “serious” versus “life-threatening” in terms of adverse events because I could not find it anywhere in the studies I looked at. Given they are classifying a COVID-19 pneumonia as “non-severe COVID,” I am not putting anything past them and assuming they just accepted the FDA definition. And we have no idea how broadly or narrowly they applied “Disability or Permanent Damage” if they did use that as part of its definition for “serious.”
Also, Pfizer does not distinguish between serious and severe COVID-19 in the six month report. It lumps them up into one category in accordance with the FDA defined protocol. The CDC definition is more enlightening in this regard, but that data is not available in the preprint. This is interesting in that the original FDA guidance document suggested separating out critical and severe COVID cases (see pages 17 and 18) so I am a little puzzled why they changed this position as that would make things much clearer.
Under the FDA protocol, a person could have a pulse oximeter reading of 92% at rest with no need for a hospital or ICU admission or other progression of symptoms and be counted as “severe.” And certainly that is “severe” in terms of intensity, it is just not necessarily serious, critical, or life threatening. We have no idea how many of these 30 cases are severe vs. critical, although the CDC criteria on the label suggest that for those that are not, there could be at least an equal number added that are due to a hospitalization.
Now lets take a severe adverse event like was suffered by actress Sally Kirkland, who has experienced five months of the most severe pain she has ever suffered in her life since right after she got the second jab. If I were a betting man, I would say that would be classified by the Pfizer scientists as severe and not serious since they could justify a milder classification with something like “there is no evidence at this point this is an actual disability or permanent damage; she can still walk can’t she?, maybe this goes away…” The point I am trying to make is that some of this is very subjective, and the investigators who are making the determination are being paid by the pharmaceutical company. The drug manufacturers investigators have an incentive to minimize every side effect to a lower category whenever they can since if there is a side effect it is more likely than not to be in the treatment arm since you do not expect placebos to have adverse events that are not random chance (at least, if it really is a placebo…).
I believe that such an adverse event would be labeled “severe adverse event” and “not serious” in a study like this as it is not life threatening and the disability would likely be considered minimal by a pharmaceutical investigator (Pfizer/BioNTech/FDA, prove me wrong by releasing ALL the adverse event data). It would not be classified as an individual disease that is highlighted because they have no idea why exactly it is happening. It is a reaction unique to her, and would not even be mentioned because it is an n of perhaps 1 in a sample of over 21,000 if she were in the study. It would simply appear as an inconspicuous +1 in the category of “severe adverse events” assuming that is how it was classified. Severe adverse events that do not even appear on the label. And the only way you could distinguish between this event being potentially vaccine related or a multiple standard deviation “coincidence” is a statistical separation in this class of adverse events which is EXACTLY what you see here with the 68.6% increased risk in the vaccine arm. So to all those patients having their side effects being dismissed as not vaccine related, here is where you might want to look.
And I do not know about you, I would prefer to have an ICU encounter that I recovered from, a relative short stint of pain and discomfort, than a prolonged 5 months and possibly the rest of my life in severe pain. Some might even prefer death. But certainly everyone would rather have this severe adverse event than a severe COVID-19 case that is just a pulse oximeter at 92 and no other problem.
If you look at the VAERS database as well as web forums and Telegram groups not censored by social media, the anecdotal reports of severe life altering side effects are significant. So it is really hard for me to believe given there was nearly an exact match in the serious side effect profile on the label (268 in both arms, 103 in 16-55 + 165 in 56+ in the vaccinated arm and 117 in 16-55 + 151 in 56+ in the placebo arm, see page 13 of the label below) that Pfizer took anything but a very narrow view on what comprises a serious event and pushed all these events into “severe.” In the 6-month preprint the serious adverse events were nearly cut in half with 127 in the vaccinated arm versus 116 in the placebo arm, which is a bit more concerning for the vaccine. There are all over 16 years of age patients so I have no idea why this is happening unless there was some huge bump in adverse events in the short time between these reports, which I find hard to believe. This is another reason I would have liked to have seen them define what a “serious adverse event” is rigorously. I have a sinking suspicious the definition is completely different between the label and the six month report preprint. This large unexplained discrepancy alone should make you question how much wiggle room the investigators have in slotting patients into these categories.
Source: COMIRNATY Package Insert, Page 13, Serious Adverse Events.
But until we have full transparency on the data, all we can do is classify like with like: “severe” with “severe” recognizing there will be some severe COVID-19 outcomes that are worse than some severe adverse event outcomes and vice versa. I think it would be the best interest of everyone for all adverse events to be spelled out in more detail in some document made available to the general public so we can judge for ourselves whether the classification qualitatively makes sense. If the FDA is allowing Pfizer to avoid classifying a COVID-19 death as “severe” then they need to allow a lot more transparency in how adverse events are treated.
What we do know, is you are 41.6% times more likely to have such an outcome if you take the vaccine, and that is not a statistical fluke. And that is only one of the many reasons that people should think twice before volunteering to be a human guinea pig by taking these vaccines.